- Plenty of Room
- Posts
- RNA Nanocages Breakthrough: ROOL Is Nature's RNA Origami!
RNA Nanocages Breakthrough: ROOL Is Nature's RNA Origami!
Uncovering the RNA nanocages that bacteria build, and what we could do with them
Cryo-EM is revolutionizing structural biology, letting us see and study biological systems like never before! And today, we look into some cool, new, all-natural RNA nanostructures!
Share this issue, it helps us grow!
Was this email forwarded to you? Subscribe here!
Nature’s RNA Origami
Scientists discovered that the ROOL RNA forms a whole natural RNA nanocage that could be used in RNA drug delivery. Image credit: Nature.
When we think of RNA, it’s usually about what it does: mRNAs are involved in translation, microRNAs downregulate the expression of specific proteins, and guide RNAs in CRISPR systems, well, guide the proteins.
But RNA is a versatile molecule! And it's more than just its functions: it’s also about form.
For example, ribosomal RNAs create complex 3D structures to interact with proteins and allow translation. Transfer RNAs (tRNAs) blend structure and function, bridging codons and amino acids.
But these long, non-coding RNAs are not alone.
Scientists have dug out hundreds of other non-coding RNAs in bacterial genomes, with around 20 long ones (>200 nt). But what do they do? How do they look? We don’t know!
Take ROOL (Rumen-Originating Ornate Large) RNAs. These 600-nt-long RNAs were first found in metagenomic data from the cow rumen, and then they popped out in bacteriophages and human pathogens. Or GOLLD RNAs, which are even longer at 800 nt!
These RNAs are often found close to genes for tRNAs and in prophages (when a phage integrates its genome into a bacterium). So, they might be involved in phage-host communication or RNA-based regulation. These RNAs even have high expression, but knocking them out doesn’t seem to affect the bacteria. Bizarre!
So, what better way to understand something than to see it?
Cryo-EM to the Rescue
The author of today’s paper used cryo-electron microscopy (cryo-EM) to study the structure of two versions of the ROOL RNA. The first one is ROOLEfa from Enterococcus faecalis, and the second one is ROOLFirm from a Firmicutes bacterium.
Enterococcus faecalis is naturally present in the human microbiota, but it can also be pathogenic, especially in hospitals, causing urinary tract infections and even endocarditis! Firmicutes is one of the most abundant bacterial families in the human gut microbiota.
ROOL: Nature’s RNA Nanocages
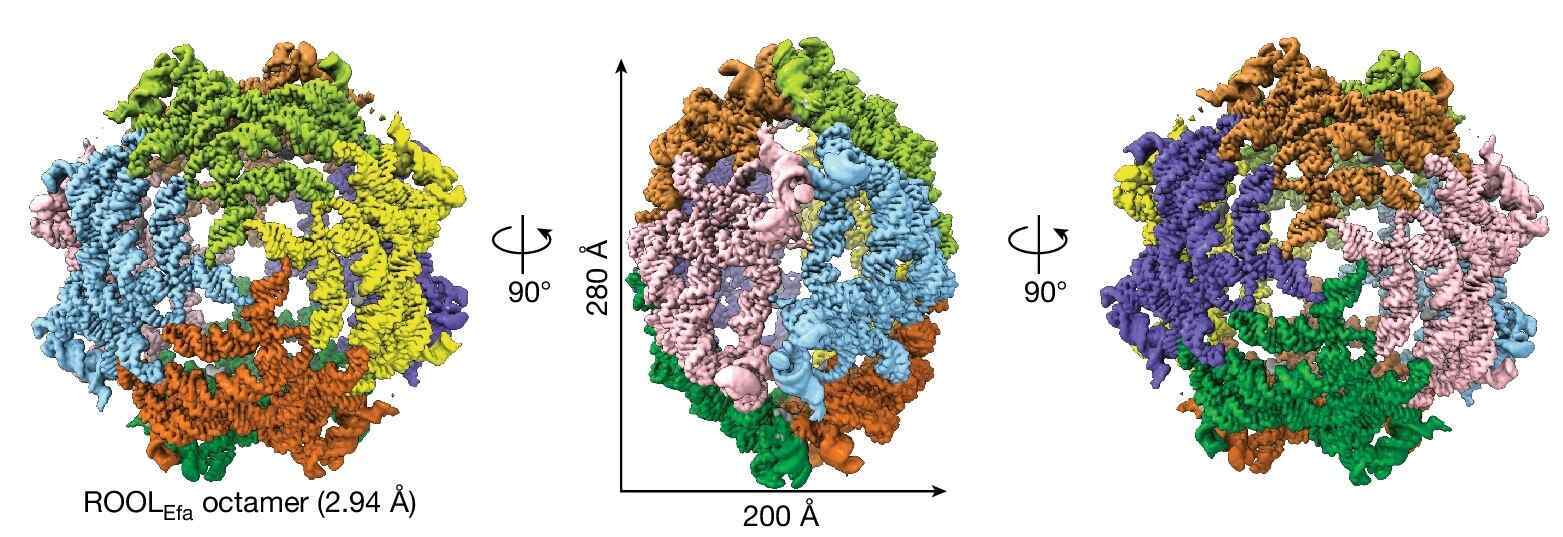
The ROOL RNAs fold into nanocages! Imagine a hollow sphere with a diameter of 28 nm and a length of 20 nm, built by eight RNA monomers interlocked. They remind me of RNA origami structures!
The team resolved the structures for :
ROOLEfa at 2.94 Å resolution
ROOLFirm at 2.93 Å resolution
The isolated ROOLEfa monomer at 3.25 Å resolution
The resolution is high enough to understand how this intricate structure assembles!
Architecting a Nanocage
Each nanocage is made from 8 monomers, arranged into 2 tetrameric (4 monomers) hemispheres.
Each monomer has 16 helices, stabilized by several interactions:
Kissing loops: short, complementary loop–loop base-pairing
Triple-strand A-minor interactions, especially A-minor staples: these are newly coined to describe three-helix junctions held together by stacked A-residues
Z-anchors, base triples, 90° turns, and ribose zippers
These motifs mediate both intra- and inter-molecular contacts, and they are crucial for the integrity of the cage.
The team even proposed a mechanism for nanocage assembly!
The monomer doesn’t look the same when it’s free versus when it’s inside the cage. When the monomers meet, a mechanism called strand swapping comes into play. Two helices from different monomers lock into each other, causing a change in conformation and aligning helices to form kissing loops between different monomers.
The assembly probably proceeds in steps: monomers → dimers → tetramers → octamer. The team detected all these different species using cryo-EM and mass photometry!

The ROOL RNA monomer. Pretty complicated, eh? Image credit: Nature
Functional Flexibility and Environmental Sensitivity
Inside the cage, the team discovered disordered regions, appearing as low-resolution loops. To see if they were needed, the team removed them. But the cages still assembled! Could they be needed for cargo interactions? Who knows!
What we do know is that the assembly of the nanocages is ion-dependent:
Higher concentrations of K⁺ and Mg²⁺ favor the formation of the octamer
Lower concentrations push towards monomers
This suggests that ion conditions (and maybe additional molecules?) regulate assembly/disassembly inside the cells!
Nanocages as RNA Delivery Vehicles
Okay, so we don’t really know what the ROOL nanocages do in bacteria.
But! This didn’t stop the team from repurposing them for RNA delivery. RNA drugs are promising for various medical uses, from vaccines to cancer treatments and guide RNAs in CRISPR-Cas systems.
The team fused functional RNAs to the ROOLFirm monomers, successfully producing nanocages with 8 copies of cargos:
Mango-III aptamer: A fluorescent aptamer used for imaging
tRNA precursors: Used in gene therapy!
MicroRNA: can be used to downregulate gene expression
A Nano Oddity with Big Potential
Always super cool images in structural biology papers! And this one was no exception. It’s amazing how much information researchers can get from just models.
So, in synthesis:
Natural functions:
We don’t know the biological roles of ROOL and similar RNAs. But the structure and the position in the genome hint at:RNA/protein encapsulation or transport
Possible virus-host interactions
Structural roles in bacterial or phage RNA metabolism
Engineered Applications:
ROOL nanocages are:
Modular and stable
Easy to engineer
Multivalent: they can easily display more than one copy of a cargo!
They could be turned into natural RNA-based nanocarriers. RNA structures are stable, biocompatible, and specific, so there is potential here!
So, if you want to see more amazing pictures of this new class of natural RNA nanostructures, just head here!
If you made it this far, thank you!
What do you think? Could this be the next big thing in therapeutics? Or do you see other uses? Reply and let me know!
P.S: Know someone interested in RNA delivery? Share this with them!
More Room:
More Assembling, Fewer Problems: It’s always great to see more ways to assemble DNA nanostructures! This study introduces a strategy for assembling DNA nanostructures using hydrophobic interactions. By attaching small hydrophobic molecules to DNA strands and integrating them into precise sites, the team enables programmable, higher-order assembly beyond traditional base pairing. Nice!
Mixing DNA with Peptides: You know what’s better than one new assembly mechanism? Two assembly mechanisms! This study enhances DNA nanostructure-based drug delivery by using a dual-function cationic peptide instead of magnesium to drive self-assembly. The peptide improves cell targeting, membrane penetration, and stability, enabling efficient delivery of doxorubicin and KRAS siRNA. In a mouse tumor model, the peptide–DNA platform showed strong tumor targeting and anticancer effects, highlighting the promise of peptide-enhanced DNA nanomedicine.
Assembly by Ligation: Okay, I promise next week it will not only be DNA nanostructures assembly! This study uses ligation as an active driver of DNA nanostructure assembly, not just for post-assembly stabilization. By ligating transiently paired DNA segments, the method converts weak interactions into permanent bonds, enabling the formation of both discrete and hierarchical superstructures.
Share Plenty of Room with founders or builders
I help biotech and deep tech companies transform complex technologies into engaging content that builds credibility with investors, partners, and potential hires. Let’s chat!
Know someone who’d love this?
Pass it on! Sharing is the easiest way to support the newsletter and spark new ideas in your circle.Got a tip, paper, or topic you want me to cover?
I’d love to hear from you! Just reply to this email or reach out on social.