- Plenty of Room
- Posts
- AI-Designed Protein Targeting: Taming Disordered Protein Chaos!
AI-Designed Protein Targeting: Taming Disordered Protein Chaos!
How computational design is unlocking targets once thought undruggable
Disordered proteins bring chaos to cells: flexible linkers, disordered tails, and a tendency to be involved in everything, from metabolism to cancer. They have been hard to target, but today’s paper changes that, with help from computational methods (and yes, a bit of AI).
Do you want to be a helper? Share this issue today!
Was this email forwarded to you? Subscribe here!
🙏 I need your help! What else would you like to read? Please fill out this form to win some karma points and help me get you better content!
Taming Disordered Proteins
Most proteins like to fold into nice and neat structures. I’m sure you’ve seen an alpha helix or a beta barrel, like the ones in GFP. But not all proteins are so well behaved! Some opted for chaos.
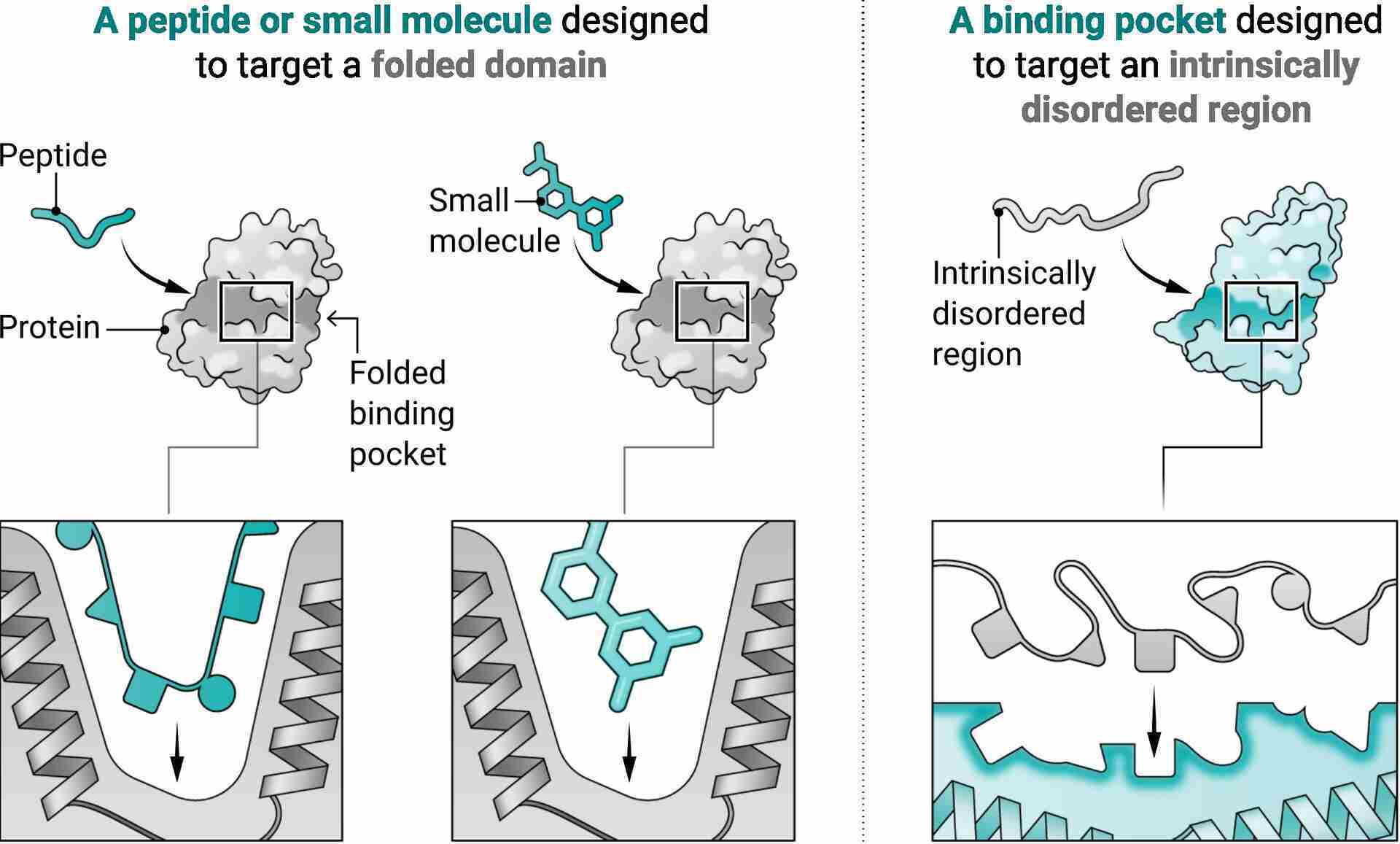
Intrinsically disordered proteins (IDPs) don’t have a fixed 3D structure.
IDPs are common in eukaryotes, where around 30% of proteins have long disordered regions and more than 70% have disordered tails or flexible linkers! And these proteins are involved in many diseases, ranging from neurodegenerative diseases to cancer and chronic pain.
IDPs: Hard to Hit
But targeting IDPs is hard.
Our traditional methods, based on knowing the target’s shape, don’t work on them. Nature has some ways to target them, from natural antibodies to repeating peptides and Armadillo repeats (yes, these are a thing). But we still have trouble creating specific binders! We have to rely on slow and expensive techniques like immunization and library selection.
Current computational methods have given us some binders, but they don’t generalize. The regions in these proteins shift conformations, creating different structures, and often don’t offer the typical binding surfaces you’d find in structured proteins!
Is there a solution here?
Merging Physics and ML Models
Here is where today’s paper comes in. The authors asked: can we design high-affinity binders for IDRs in a generalizable way?
They decided to combine the strength of two complementary design approaches:
Physics-based models: Like Rosetta, they have been used to create binders with repeated regions, with each unit interacting with a part of a target. This reliance on structure limits their ability to create binders for variable, disordered regions!
Deep learning models: Like RFdiffusion, they have been used to create binders without structural constraints. These models start with a “cloud” of amino acids, turning it more and more into a real molecule with each iteration. But they generally create stable structures, incompatible with disordered regions!
But what if we were to merge the two approaches?
The Design Pipeline
This is where things get cool. And a bit complicated.
I’ll be honest with you, I found it hard to understand their method, but I got there eventually.
To make it simple, they first built a library of protein scaffolds with tiny “pockets” that can bind to amino acids in flexible disordered regions. Then, they mixed and matched these pockets to match the shape of a chosen target sequence.
Okay, now let’s get into the nitty-gritty!
1. Generate a Library of “Pocket” Scaffolds
This part is divided into:
Scaffold generation
Rosetta builds repeat proteins that wrap around repeating units on peptides. These repeating units form a binding pocket, with the side chains interacting with the targets.Pocket specialization
These binding pockets are fine-tuned using RFdiffusion to obtain binding to specific peptides!Pocket assembly
Finally, RFdiffusion recombines the pockets in new orders/geometry, binding to different regions!
The result is a library of 1,000 binder templates, tuned to interact with disordered sequences from 8 to 40 residues.
2. Thread and Refine: Matching to Targets
With the library ready, now you can “thread” target sequences through all the binders in their library to find one whose pockets align well with the target’s amino acids.
The pipeline then fine-tunes the binder’s sequence (using ProteinMPNN) and filters using the structures predicted by AlphaFold2. Additionally, the researchers applied RFdiffusion again to optimize some of the binders.
The best candidates were then selected and tested experimentally!
The Results: Binder for IDPs
The team tested their method on 43 targets, including synthetic sequences and clinically relevant IDPs or disordered regions.
The results?
They got hits for 39 of them, with an average of just 28 designs tested per target!
Many had nanomolar affinities
Several induced structural changes in their disordered targets upon binding
Some binders bound to very polar or “undruggable” IDRs that would defeat normal design approaches
The researchers solved the crystal structure of a binder together with dynorphin, a ligand implicated in chronic pain. The structure matched the design, and they also noticed that the disordered regions in the dynorphin molecule became more ordered after the binding!
But they decided they weren’t done.
Applications of Designed Binders
Binders like these have obvious uses as drugs, but they can also be useful in other contexts!
The team demonstrated this by applying the binders to solve some problems:
Protein enrichment:
A lot of important proteins are present in low quantities. They used their binders to capture some of these proteins and flush out anything they didn’t need, getting better results!Cell-surface targeting:
One of the binders was used to bind a cell protein upregulated in many cancers. This could be useful to target cancer cells more effectively!Mitochondrial targeting:
They showed that only the right binder binds a mitochondrial target, showing off the specificity of the designs.Functional inhibition:
The binder for dynorphin we talked about before? The only known inhibitor for that protein!
In Conclusion
Great work! The team took advantage of the heterogeneity of disordered proteins, a smart move!
In stably folded proteins, you might have a few optimal binders, but here, a variety of conformations can work! So sampling a library is a simple (and smart) strategy. This allowed them to target challenging proteins.
I also found it interesting that many of the binders induced changes in the disordered regions! Just a cool thing, I wonder how it could be helpful. Maybe for better crystals?
Their method is also computationally efficient, and with only 22 designs per target, it has a high success rate. The combined power of physics-based models and machine learning!
And the applications are infinite: cancer, neurodegenerative diseases, chronic pain, viruses… I mean, 70% of proteins have disordered regions!
This was a bit of a tough read, and there are details I had to leave out. So, go and read it here!
If you made it this far, thank you! What do you think of this method? Do you think that this combination of physics and AI-based models is working? Reply and let me know!
P.S: Know someone interested in AI-based protein design? Share this with them!
More Room:
Protein Contacts and Networking: Even proteins are not immune to networking, damn. This study uses cryo-EM to explore how membrane proteins form higher-order transient clusters, which help coordinate signaling pathways. The authors show that some proteins cluster via structured domains, while others use intrinsically disordered regions. These different interaction modes lead to clusters with distinct levels of compactness and organization, revealing the diverse strategies nature uses to assemble membrane signaling hubs.
Dox, DNA Origami, and Fluorescence: Is there a more iconic duo than DNA origami and doxorubicin? This study uses fluorescence anisotropy to better understand how doxorubicin (DOX) binds to DNA origami nanostructures, a promising cancer drug delivery platform. The authors show that DOX binding depends heavily on loading conditions: at higher ratios, DOX tends to aggregate on the DONs, which may affect performance. Their findings highlight the importance of precise characterization to ensure purity and efficiency of DOX-nanostructures complexes, offering a more informative method than traditional fluorescence intensity measurements.
Super-Resolution for the Win: Super-resolution is super cool, and it has helped elucidate lots of mechanisms in biology. This article proposes a unified framework for defining and measuring resolution in super-resolution microscopy (SRM), aiming to clarify its true capabilities for structural biology. By addressing the diversity of SRM techniques and resolution definitions, the author highlights what current methods can realistically achieve, potentially reaching ångström-scale resolution, and outlines future directions for live structural imaging.
Share Plenty of Room with founders or builders
I help biotech and deep tech companies transform complex technologies into engaging content that builds credibility with investors, partners, and potential hires. Let’s chat!
Know someone who’d love this?
Pass it on! Sharing is the easiest way to support the newsletter and spark new ideas in your circle.Got a tip, paper, or topic you want me to cover?
I’d love to hear from you! Just reply to this email or reach out on social.