- Plenty of Room
- Posts
- DNA Origami Needles: Virus-Inspired Nanostructures for Drug Delivery!
DNA Origami Needles: Virus-Inspired Nanostructures for Drug Delivery!
Engineering Virus-Like Delivery Using Programmable DNA Structures
Viruses killing bacteria, bacteria killing viruses.
It’s an arms race as old as life itself! And it gave us fundamental insights into biology, CRISPR-Cas systems, and probably more I’m forgetting.
Can we now use the virus structure itself as an inspiration for new drug delivery options? Well, DNA origami is happy to lend a hand!
Don’t keep this newsletter a secret: Forward it to a friend today!
Was this email forwarded to you? Subscribe here!
Viruses, DNA, Needles
Researchers created virus-inspired DNA origami nanostructures that deliver small molecules directly into the cell cytosol. Image Credits: Advanced Science.
Viruses: Infecting Everything
Viruses are the most abundant biological entities on Earth.
Neither alive nor dead, they infect everything. And I mean it: plants, animals, bacteria. In the ocean, there are 10 viruses for each bacterial cell! And trust me, there are a lot of bacteria in the ocean.
Most scientists don’t consider viruses a life form. They have some characteristics of life, but not all of them, and as usual in science, people disagree. I don’t have a horse in this race, but I do think that viruses are cool!
Viruses are big in delivering their genetic material into host cells. Well, it’s their whole thing: they can’t replicate on their own (you see where the disagreement comes from?). And over billions of years, they have evolved an incredible toolbox of entry strategies!
In animal cells (like yours and mine), viruses hijack membrane receptors and let the cell do all the work. Once inside, they escape into the cytosol and start wreaking havoc.
Plant viruses have to fight the rigid cell wall. So, they wait for wounds or jump into an insect, bypassing the wall entirely and fusing directly with the membrane to release their nucleic acids into the cytoplasm.
But bacteriophages have the coolest mechanism, hands down.
Bacteriophages: Direct Injection
Bacteriophages infect and replicate inside bacteria.
There are an unreasonable number of species, each with its own adaptations. They infect all different kinds of bacteria, after all! And the entry mechanisms are also wildly different.
But T4 bacteriophages stand above the rest.
T4 has been a central part of biology for nearly a century. Helping answer molecular biology questions, enabling phage display, and even getting Nobel prizes! When you think of a phage, you’re thinking of T4, trust me.
Their little icosaheadral head, the tail, the almost-insect-like legs… You know what I’m talking about! Looks almost cute. A weapon of biological murder, but still cute.
T4 uses its legs to anchor to the bacterial cell. The tail pierces the cell wall and the membrane, helped by degrading enzymes. The DNA rushes through the hollow tail and is injected directly into the cytosol, where it will take over the whole bacterium.
Brutal. An extremely effective weapon, honed through millions of years of evolution!
So, scientists (still a bit shocked) asked: Can we use it for drug delivery?
Inspired by Phages, Built From DNA
Scientists have always looked at nature for inspiration. And now, we can create our own nanoscale machines.
Today’s paper does just that.
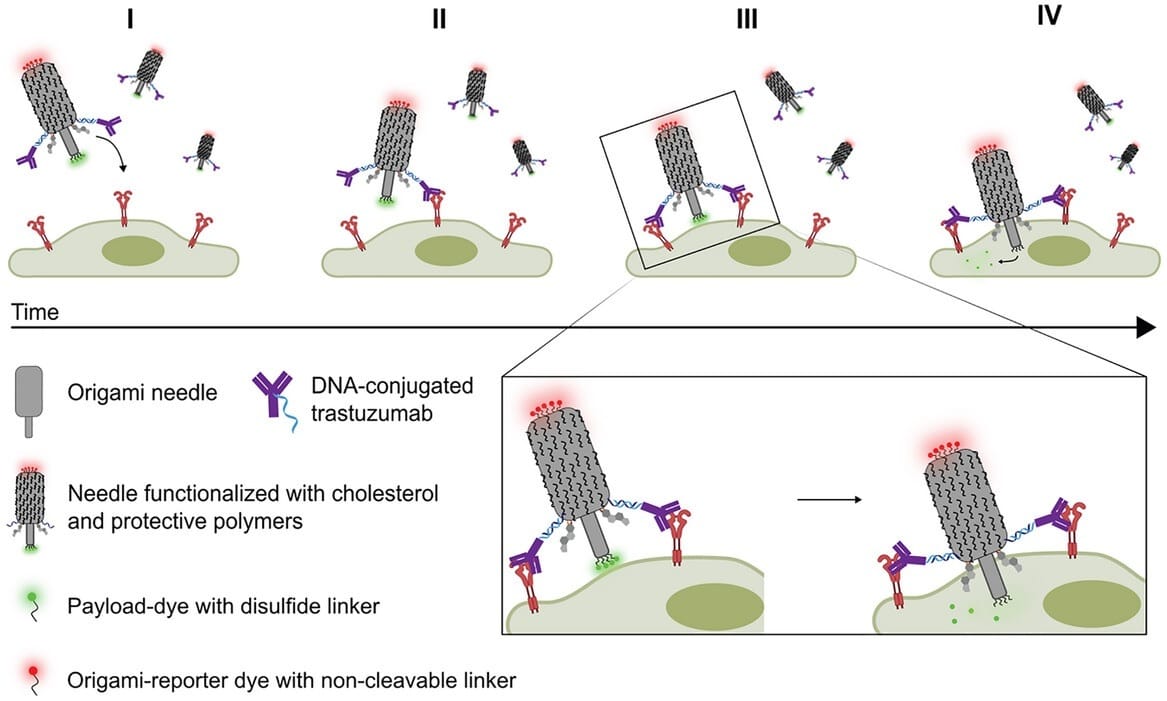
The authors created a bacteriophage-inspired DNA origami “needle” for payload delivery. It binds HER2 on cancer cells, uses cholesterol anchors to pierce membranes, and releases small molecules directly into the cytosol!
An autonomous, selective nanorobot for targeted drug delivery.
DNA Origami Needles: Design and Fabrication
DNA origami is the most powerful technology for nanoscale self-assembly.
Okay, I might be biased, but that’s not far from reality. DNA origami uses a long, single strand of DNA as a scaffold, together with a bunch of smaller “staple” strands, to create custom nanostructures!
And they have many advantages:
Biocompatibility
Nanoscale precision
Incredible programmability!
So, they are cool. And you can find them from drug delivery to nanoscale printing and synthetic biology!
Two modules form the needle DNA origami:
A barrel-shaped base, around 50 × 20 nm
A thin, 12 nm-long needle stem
That’s the skeleton, but more functions must be added. Worry not, the programmability of DNA lets you attach anything to it!
In this case, 4 functionalization modules:
DNA-conjugated antibodies (Ab-DNA) for targeting. 2 copies of DNA-trastuzumab target HER2, a membrane receptor overexpressed in cancer cells.
Cholesterol-modified DNA strands (17, to be precise) drive membrane insertion.
The disulfide-linked payload is attached to the tip of the needle.
To survive physiological conditions and cell media, the structures are coated with a biodegradable polymer (PCD).
All this turns a humble DNA origami into a synthetic bacteriophage!
Here’s the idea:
Targeting: The Ab-DNA binds HER2 on target cells, ensuring selectivity.
Insertion: The cholesterol anchors enter the lipid bilayer and drive the needle through the membrane.
Payload release: High intracellular glutathione (especially in cancer cells, another mechanism for selectivity) cleaves the disulfide linkers and frees the small molecule payload into the cytosol.
Awesome! Elegant, sophisticated, and effective.
To the Bench: Does it Work?
First, the authors confirmed that everything assembled correctly using the classic DNA nanotech toolbox. Agarose gels, TEM, and stability assays confirmed the structure formed; insertion into synthetic liposomes confirmed its function!
But the real test: Does it work in cells?
The team focused on HER2-positive breast cancer cells, an aggressive form with high clinical relevance.
Flow cytometry and confocal microscopy showed strong, antibody-dependent signals both from the membranes and the cytosol of the HER2- expressing cells. This signal disappeared in lower-HER2-expressing cells! And the structures showed no toxicity.
To better study the system, the authors directly tracked the payload fate at single-molecule resolution.
They added SeTau647 fluorescent dyes to the tip of the needle, with a cleavable linker. Using HILO microscopy, they tracked the payload and the cellular membrane at the same time.
Comparing the full nanostructures with no-antibody or empty controls, the team saw that the full needle produced many trackable payloads that migrated into the cytosol. In contrast, control structures were mostly stuck at the membrane.
In short, antibodies are essential, and there is real cytosolic delivery!
The Advent of Needles?
Such a cool idea! I had a similar one back in my PhD days. Very interesting!
I can see strengths:
Modular, biomimetic design: It combines antibody targeting, cholesterol-mediated insertion, and stimuli-responsive release (glutathione). And all these can be swapped around, for other targets or mechanisms!
Stabilization strategy (PCD): The polymer coating makes the origami usable in physiological conditions.
Single-molecule evidence of cytosolic delivery! It’s not every day you come around such a well-studied system.
Of course, there are limits:
Cargo size: How do you deliver larger therapeutics, like mRNA or antibodies? I don’t know. But for the moment, the needle tip can work for small molecules, dyes, oligos, or small proteins!
In vivo translation: It will be cool to see if something like this works in vivo! I’m excited to see more DNA origami applications.
Delivery mechanism: How does the structure work? Does it form a pore? Is the membrane remodeling? What happens to the payload once it enters? Could be important to know for therapeutic applications!
But an awesome read, with a high level of detail! I’m always so excited to read about new, cool DNA origami research.
Get all your details (and cool images) here!
If you made it this far, thank you! What do you think of these giant DNA origami needles? How do you think they compare to more standard techniques? Reply and let me know!
P.S: Know someone interested in DNA origami and SynBio? Share it with them!
What did you think of today's newsletter?Your feedback helps create the best newsletter possible! |
More Room:
New Gene Coordination Just Dropped: And it’s a big update. Gene co-expression at the RNA level is well studied, but whether translation is coordinated across genes has been unclear. In this study, the authors analyze 3,800+ ribosome profiling datasets from human and mouse cells and introduce translation efficiency covariation (TEC), a measure of coordinated translation across conditions. They show that TEC is conserved between species, reveals functional gene relationships not captured by RNA or protein co-expression, and is enriched among physically interacting proteins. The work establishes TEC as a fundamental principle of translational regulation with potential applications in gene function prediction and synthetic biology.
Peptide Codes for RNA Therapies: RNA therapies promise to solve many diseases. I mean, it did help with COVID! Efficient mRNA therapies require delivery beyond the liver, but current lipid nanoparticle (LNP) strategies struggle to target other organs. In this study, the authors introduce peptide-encoded organ-selective targeting (POST), a modular approach that decorates LNP surfaces with specific peptide sequences to direct extrahepatic delivery. They show that organ selectivity arises from peptide-driven protein corona formation, which tunes LNP interactions with plasma proteins and tissues. The POST platform enables organ-selective delivery of mRNA and gene-editing cargos, expanding the versatility of LNP-based therapeutics.
A Birdseye View of De Novo Designed Binders: It’s hard to keep up with science in general. Keeping up with de novo protein design? That’s a job in itself. This helpful review brings you up to date, from the origin of the field to today. Today, researchers can rapidly design de novo protein binders with tailored structures and high affinity for difficult targets, greatly reducing development time and cost compared to traditional methods. Recent successes include binders that neutralize toxins, modulate immune responses, and target disordered proteins. As models continue to improve, AI-driven binder design is emerging as a powerful paradigm for programmable protein engineering and therapeutic development.
Share Plenty of Room with founders or builders
I help biotech and deep tech companies transform complex technologies into engaging content that builds credibility with investors, partners, and potential hires. Let’s chat!
Know someone who’d love this?
Pass it on! Sharing is the easiest way to support the newsletter and spark new ideas in your circle.Got a tip, paper, or topic you want me to cover?
I’d love to hear from you! Just reply to this email or reach out on social.