Welcome to Plenty of Room!
Today, we are taking a close look at mitochondria and ATP synthase and then we are talking about mice brushed with… Doritos? Well, let’s jump into it!
Plenty of Room is your guide to the cutting-edge news related to molecular machines. New here? Just go ahead and subscribe! Already subscribed? Help a friend or a colleague save some time and share this with them!
Let’s get into it now.
A Closer Look at ATP Synthase
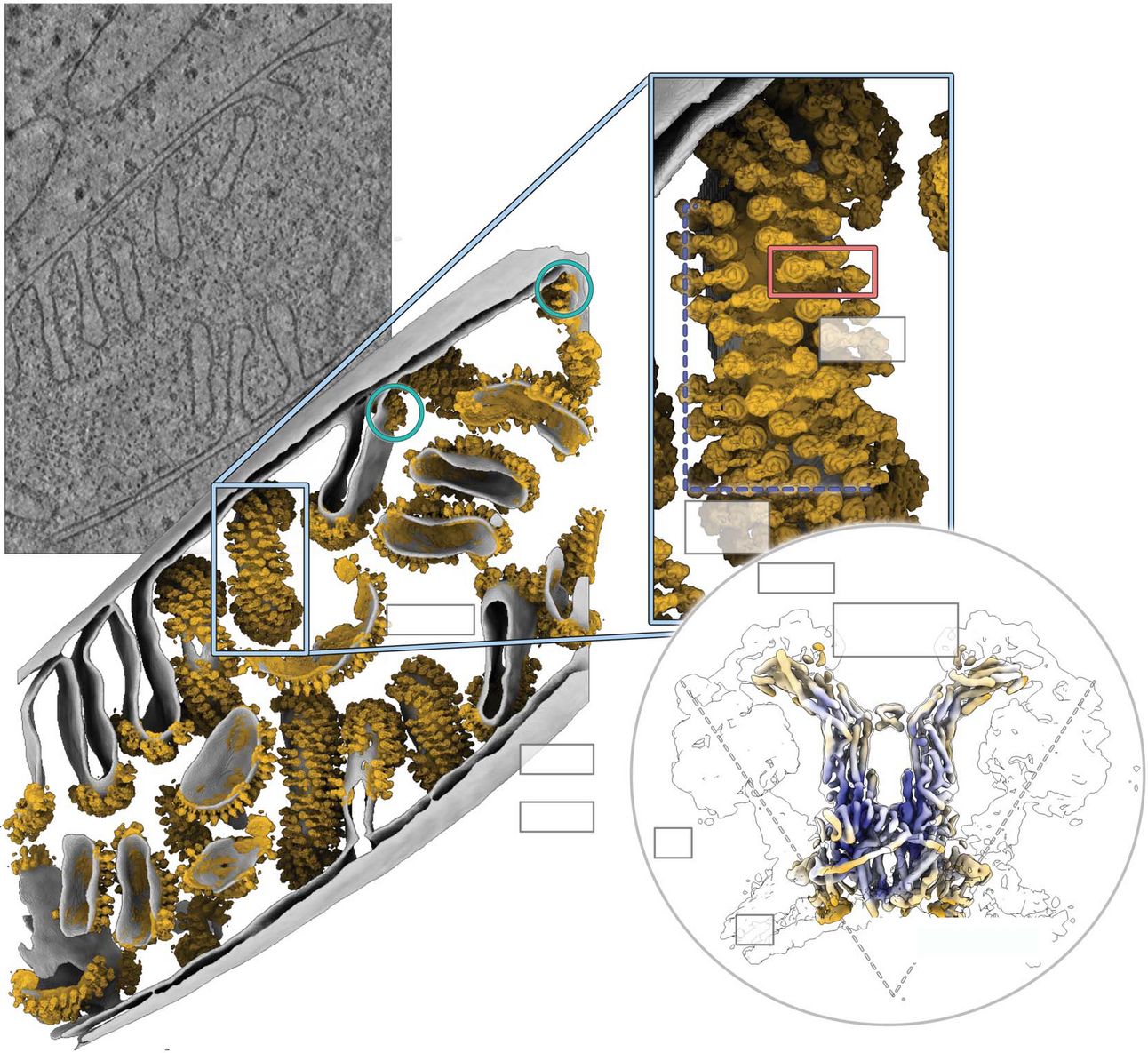
In situ structure of the mitochondrial ATP synthase. From Lea Dietrich et al. Science. (2024)
I really love structural biology papers: they always have the coolest figures! So, today we are looking at fascinating paper that uses electron cryo-tomography to study proteins directly within the mitochondria of Polytomella, a unicellular flagellate.
Mitochondria and ATP synthase: a Long Love Story
Ah, the mitochondria: the powerhouse of the cell we all know and love. This organelle is in charge of generating most of the chemical energy of the cell, using aerobic respiration to generate adenosine triphosphate (ATP). The star of the show is the ATP synthase complex, a molecular turbine that sits in the mitochondrial membrane, harnessing the energy of the proton gradient to drive the rotation of parts of the enzyme, creating ATP in the process.
The ATP synthase complex consists of two primary subcomplexes:
F1 head: The catalytic engine, located inside the mitochondrial matrix.
Fo subcomplex: The membrane-embedded section that is responsible for proton translocation and torque generation.
These subcomplexes work together, with the Fo region generating torque to rotate the central stalk, which in turn powers ATP synthesis in the F1 head.
Studying ATP Synthase in Situ
Now, what's special about this study is that, unlike previous research that isolates and purifies proteins for analysis, the authors chose to study ATP synthase in situ, within its native cellular environment (well, after freezing and milling it, I guess). This approach can preserve crucial interactions that may be lost during the isolation and purification of the protein, offering more accurate insights into the functions of proteins.
So, what did they find? Well, first of all, they were able to discover that In Polytomella cells, ATP synthase forms left-handed helical arrays that run along the cristae ridges of the mitochondrial inner membrane. These helices are made up of ATP synthase dimers (two ATP synthase molecules) and this arrangement helps stabilize the disk-like cristae. It may also help create local proton reservoirs, making the ATP production process even more efficient..
A key focus of the study was the peripheral stalk of ATP synthase, particularly in the dimers. This peripheral stalk is responsible for stabilizing the subcomplexes. With an impressive resolution of 4.2 Å, the researchers mapped key subunits of the stalk, including a previously unobserved α-helical extension on subunit ASA3. This structural feature likely needs its native environment to stay intact, which explains why it wasn’t visible in earlier studies that isolated the protein.
In addition, they were able to identify six distinct rotary states of the ATP synthase’s central stalk, with several substates for the F1 head. This rotary movement is essential for the enzyme’s function, and probably these six state represent six energy minima that the enzyme encounters as it rotates to produce ATP.
Implications and Final Thoughts
This study is fascinating and the paper has some very, very pretty figures! I would recommend it even just for that. These figures show the power of in situ structural biology: the authors managed to reach very high resolutions for their reconstructions, so that they could derive functional insights from them. These insights advance our understanding of bioenergetic complexes, that could have implications in curing disease arising from problems in the cellular energy production. In addition, studies like this could help us better optimize cellular factories for the production of various chemicals, polymers and proteins.
But don’t take my word for it, go and read the original paper!
Additional Room:
Doritos… Mice? Sometimes, science seems to work backwards. This study is one example: the authors discovered that strongly absorbing molecules can unexpectedly make live animals optically transparent. They managed to temporarily make a live mouse body transparent. The secret? The same food dye used in Doritos.
Controlling DNA Dominoes: In this work, the authors mimic allostery using a DNA origami domino array, which undergoes conformational changes triggered by specific DNA strands. They demonstrated precise control of mechanochemical reactions by releasing a cargo DNA strand at a specific step in the cascade. Pretty cool!
Merging Nanoparticles and Machine Learning: During COVID, everyone got to know about lipid nanoparticles and mRNA vaccines. In this work, the authors used machine learning techniques, to predict mRNA expression efficiency after injection in mice. This approach provides a framework for developing more efficient LNPs, advancing the potential of mRNA therapeutics, also outside of vaccination.
Not yet a subscriber to Plenty of Room? Sign up today — it’s free!
You think a friend or a colleague might enjoy reading this? Don’t hesitate to share it with them!
Have a tip or story idea you want to share? Email me — I’d love to hear from you!
You have something you would love me to cover? Just reach out here or on my social!
